An unprecedented view of protein dysfunctions in Alzheimer's disease
Alzheimer's disease is a relentlessly progressive, fatal neurodegenerative disease that afflicts more than 25 million individuals worldwide (GBD Dementia Collaborators, Lancet Public Health, 2022). Two hallmarks define the pathology of Alzheimer's disease: amyloid beta plaques and tau neurofibrillary tangles. These prominent, readily observable molecular features motivated the study of protein dysfunctions in Alzheimer’s disease. Decades of intensive research have associated protein dysfunctions, such as aberrant cleavage of the amyloid beta precursor protein, tau hyperphosphorylation, and amyloid beta and tau misfolding and oligomerization with Alzheimer's disease (Busche and Hyman, Nature Neuroscience, 2020). Collectively,this large body of evidence has suggested a critical, causal role for protein dysfunctions in Alzheimer's disease.
Studies of protein dysfunctions in Alzheimer's disease have been motivated yet also limited by what we can see. Technological challenges have precluded a systematic and quantitative understanding of protein dysfunctions that adversely impact amyloid beta and tau. As a result, the role of amyloid beta plaques and tau neurofibrillary tangles as causal factors in Alzheimer’s disease remains controversial (Karran and De Strooper, Nat. Rev. Drug Discovery, 2023). Likewise, the precise contributions of amyloid beta and tau dysfunctions to cell death in Alzheimer’s disease remain unclear, e.g., amyloid beta oligomers versus fibrils (Castro et al., Protein Science, 2019) and toxic gain versus loss of normal tau function (Ballatore et al., Nat. Rev. Neuro., 2007). More generally, we are unable to comprehensively characterize global proteomic changes in Alzheimer's and other neurodegenerative diseases. Consequently, many important protein dysfunctions, causal mechanisms, and therapeutic targets likely remain undiscovered. Until we can sensitively, accurately, and systematically measure protein dysfunctions, our efforts to understand, prevent, and cure Alzheimer's disease will be hindered.
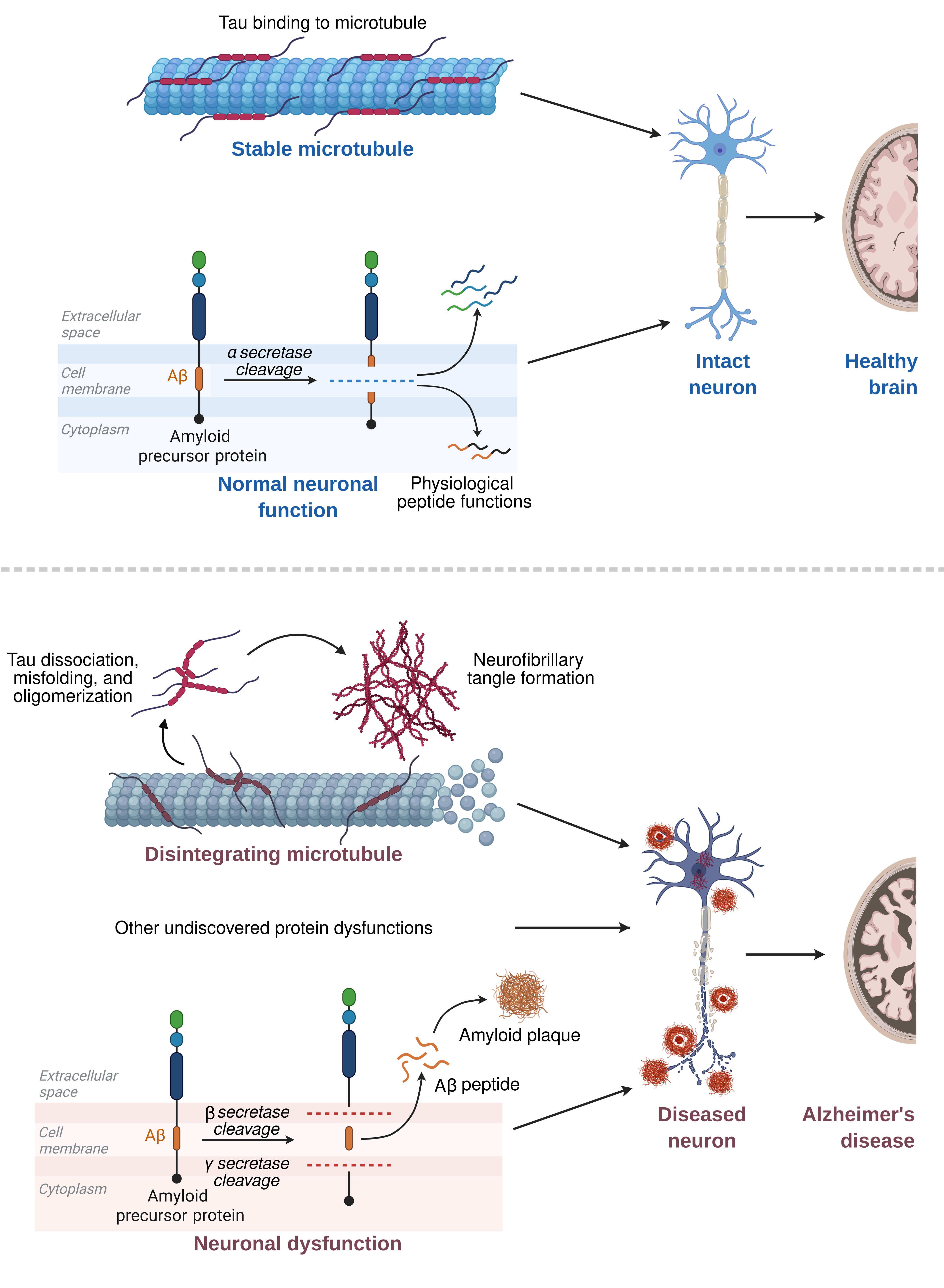
Overview of protein dysfunctions in Alzheimer's disease. Created in Biorender.
For decades, mass spectrometry has been the most powerful technology for characterizing and quantifying proteins (MacCoss, et al, Nat. Methods, 2023). Yet, its limited throughput, sensitivity and scalability severely limited its power to identify causal disease mechanisms. Our team has developed and demonstrated novel concepts, approaches, and technologies that transcend the limitations of existing mass spectrometric proteomic methods (Slavov, Science, 2020; Derks, et al, Nat. Biotech., 2023).
The Parallel Squared Technology Institute (PTI) will build upon these frameworks to overcome longstanding challenges and enable scalable, sensitive, and quantitative measurements of protein dysfunctions in Alzheimer's disease. PTI is developing new mass tags (barcodes) that will dramatically increase the throughput of mass spectrometry, which will enable large-scale, statistically powerful proteomic studies. By increasing the sensitivity of mass spectrometry, PTI will provide an expanded view of global proteomic changes. These efforts will increase the sensitivity of mass spectrometric proteomics and increase throughput 100 to 1000 fold (Slavov, Mol. Cell Proteomics, 2022)
Our team has developed and demonstrated novel concepts, approaches, and technologies that will unlock previously inaccessible views of protein dysfunctions in Alzheimer's disease (AD).
These groundbreaking advances will unlock previously inaccessible views of protein dysfunctions in Alzheimer's disease (AD). PTI is collaborating with Dr. Bradley Hyman of Massachusetts General Hospital, a recognized leader in the Alzheimer’s disease field, to develop approaches that capture global proteomic changes in single, AD-afflicted cells. These efforts will provide a quantitative view of protein dysfunctions occurring in AD at unprecedented scale and resolution. The resulting datasets will enable entirely new types of analysis that can rigorously discriminate between competing models of disease-relevant mechanisms (Slavov, PLOS Biology, 2021). The unparalleled throughput and sensitivity of our approach allows us to systematically and comprehensively explore AD protein pathologies at unprecedented scale. This exploration is likely to deepen mechanistic understanding of known AD pathologies as well as identify new, previously-inaccessible causal mechanisms and therapeutic targets.
As part of PTI's commitment to open science we will freely share our results and insights to maximize benefit to the research community, patients, and their families. We led the establishment of community standards (Gatto et al., Nat. Methods, 2023) and seek to exemplify them in our research so that everyone benefits from rigorous, reproducible science.
To learn more about the approaches PTI is using to study Alzheimer's disease, please visit our Technology and Science page.
